The gelatin composition: peptides
Gelatin has an odorless, pale yellow and translucent appearance. The moisture is around 10% to 13%, and 85% gelatin is non-complete proteins made up of 18 amino acids. The rest is a little fats, carbonhydrates, ions and ash. Similar to collagen, the G-X-Y repeating structure occupies most of gelatin, where G is glycine and XY is proline and hydroxyproline.
The molecular weight distribution is related to the raw materials and processing method, so there is no fixed value. Its molecular weight ranges from thousands to hundreds of thousands Da, and those around one hundred thousand Da is slightly more common. Even the same raw material and process, the composition will differ due to animal species and place of origin.
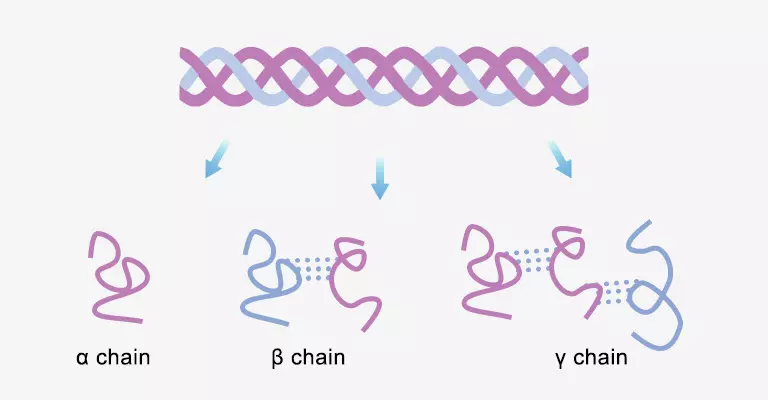
Gelatin is hydrolysis of collagen found in skin, bones and tendons. The covalent cross-linking, peptide bonds and hydrogen bonds break after being treated with acid, alkali and enzyme. Triple helix disintegration makes water-insoluble collagen into peptide soluble in hot water. The raw materials hydrolysis starts from the surface and gradually penetrates inward, thus the uniform hydrolysis is impossible. The outermost layer will be excessively hydrolyzed, while the inside will be under-hydrolyzed. Chemical bonds are broken at different sites, so there are various peptides in gelatin. They’re mainly single peptide chains called α-chains. Some α-chains will accumulate to form big molecules. They’re β-chains that has two cross-linked peptides and γ-chains that has three cross-linked peptides. Gelatin also contains a few of peptides smaller than α-chains or larger than γ-chains. Its gel strength is mainly determined by the quantity of α-chains and β-chains. The more there are, the greater gel strength. The viscosity is primarily determined by quantity of γ-chains and larger peptide chains. The more there are, the greater viscosity.
Physical Properties of Gelatin
Gelatin is soluble in hot water, but insoluble in cold water. It absorbs cold water that is 5-10 times its own weight and swells slowly. When gelatin dissolves in hot water, it is a colloid rather than a true solution due to the large molecules. Gelatin solutions begin to solidify at temperatures above 20°C. Solidification process lasts for several hours and reaches maximum gel firmness in a day or so. Gelatin gel will melt back into a solution, if it’s heated above 35°C that is slightly lower than body temperature. This infinite thermal reversibility is a unique and important property. Other hydrogels such as highly esterified pectin, agar and starch can’t revert to a liquid state once become a gel.
How Does Gelatin Solution Become Jelly?
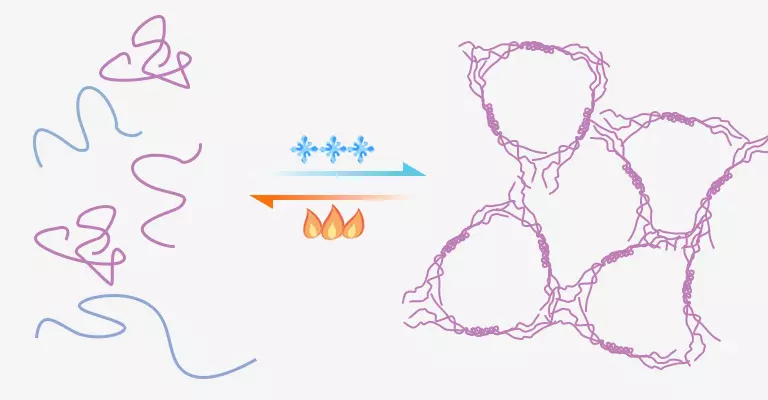
When gelatin is dissolved into hot water, these thread-like peptides move randomly and chaotically. The Intermolecular and intramolecular thermal motions result in unstable structure within the colloid. As temperature falls below a threshold, peptides entangle and aggregate to small clusters due to the decreased thermal motion. However, it is impossible to generate identical structure like collagen and just has some resemblance. Triple helices appear in certain position of the peptide chain, while other positions still resemble loose threads. The small clusters gradually grow and eventually form a net-like structure that turns the solution into gelatin gel. The intersections of molecules are net knots, and the spaces between them are meshes.
Since gelatin molecules form a three-dimensional network by hydrogen bond, van der Waals forces and hydrophobic interactions, they aren’t as strong as covalent bonds. Water molecules with a slightly larger kinetic energy can destroy this structure. The network structure reforms spontaneously as temperature decreases. This’s why gelatin exhibits infinite thermal reversibility.
Thermal History of Gelatin
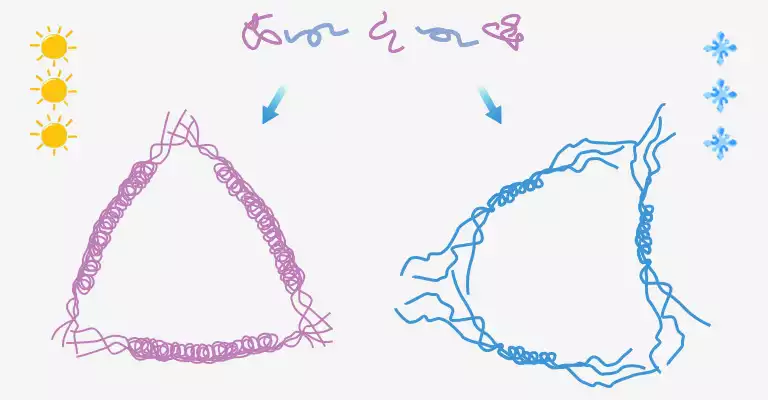
The cooling rate affects its gel firmness. If it’s quickly cooled in a refrigerator, the actual gel strength value will be lower. A firmer and more elastic gel forms as it cools slowly at room temperature.
This phenomenon can be easily explained on a microscopic level. Slow cooling allows the high temperature to break down weaker connection and what survives are long triple helices, so the slowly cooled gelatin is harder. Conversely, the shorter and instable triple helices preserved in quick cooling leads to a looser structure.
Gel strength and viscosity are important commercial parameter for gelatin
Gel strength, gel firmness or Bloom value is a critical commercial parameter for gelatin. It’s defined as the weight of a plunger pressed 4mm into a 6.67% gelatin gel under standard conditions. It measures the resistance of gelatin gel to external forces, or its hardness. In commercial activities, it’s directly associated with quality of edible gelatin whose Bloom value ranges from 50 to 300g. A higher Bloom value indicates better quality, a higher melting and setting point, and a shorter setting time. Foods made with high-quality gelatin are often more elastic and crystal clear.
Increasing the concentration or using higher-quality gelatin will enhance gel hardness. Their relationship is described by an empirical formula C₁√B₁= C₂√B₂. For example, if you want replace gelatin of 300 Bloom value with gelatin of 200 Bloom value, the concentration increases from 10% to 12.3% to achieve the same hardness.
Another important parameter that describes the molecules mobility is viscosity. A higher viscosity means that molecules face more resistance when moving in gelatin solution. Viscosity may rarely be taken into account in edible gelatin, while it is very crucial for pharmaceutical gelatin used in hard capsule. Only the appropriate viscosity ensures that gelatin solution is evenly covered the metal pins to form a film for the capsule shell.
Gelatin classification
Gelatin is classified into type A gelatin (acid-treated), type B gelatin (alkali-treated), and type E gelatin (enzyme-treated). Type A and B gelatins are common in market, while enzyme-treated gelatin is rarely produced.
Gelatin is also classified into industrial, edible and pharmaceutical gelatin by its application. Industrial gelatin is often extracted from tannery waste to reduce costs, so it doesn’t need to comply with strict food and drug regulations. Examples include matches (gelatin solution forms a porous match head), sandpaper, paper corrugated board, adhesives for furniture or high-end musical instruments, and ballistic gelatin for forensic analysis. Pharmaceutical gelatin follows the strictest rules. It has minimal metal ions and ash content to prevent reactions with the encapsulated drugs.