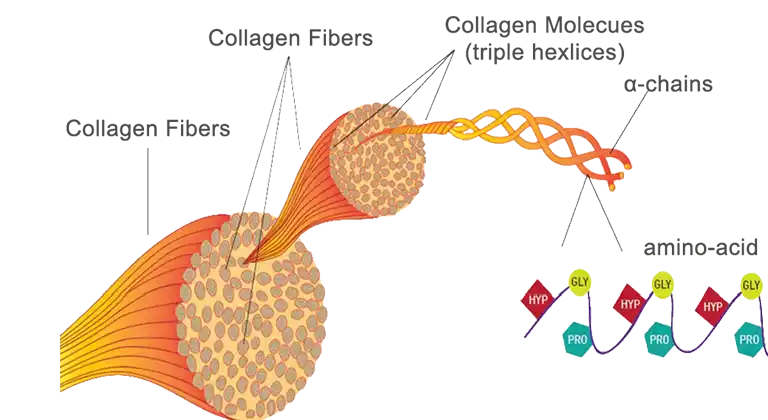
Primary Structure
Collagen is not a single protein, but a family of proteins. The collagen used for gelatin extraction is mainly type I that is abundant in the skin, bone and tendon of animals. Amino acids form collagen through dehydration reaction like other proteins. There is no repetition of amino acids in other proteins, but in collagen, there exists a G-X-Y repetitive structure that accounts for 96% of the entire peptide chain. G is glycine; proline and hydroxyproline prefer the X, Y positions, but there may also be other amino acids whose contents vary with different organisms. About a thousand of these amino acid residues make up a peptide whose C- and N-terminus are short chains without G-X-Y repetition. The lack of tryptophan and cysteine, and low content of methionine, histidine and tyrosine mean that collagen is an incomplete protein, so people cannot survive by eating only collagen.
Secondary Structure
Type I collagen consists of three peptides of which two are the same α1 peptides and the other is a slightly different α2 peptide. Each peptide is coil into a left-handed helix conformation by hydrogen bonds.
Tertiary Structure
The three left-handed helical peptide are twisted around each other to form a rope-like right-handed triple helix by hydrogen bond, called tropocollagen (300 nm in length, 1.5 nm in diameter). The groups of G are located inside the rope and the groups of X, Y are facing outwards. The space inside the rope is too small to accommodate other groups except hydrogen atoms.
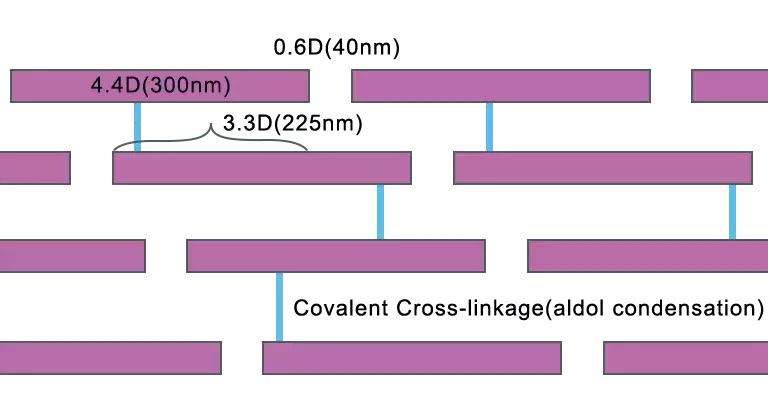
Quaternary Structure
These tropocollagen aggregate together to become fibrils spontaneously because of surface charge distribution and hydrophobic groups. In fibril, these tropocollagen molecules are staggered a quarter of their length to form multiple rows. The distance between tropocollagens in the same row is 0.6D and the overlapping part of adjacent molecules is 3.3D (D=67nm). Lysine and hydroxylysine residues of adjacent tropocollagen form covalent bonds to strengthen these fibrils. Fibrils are arranged in parallel to grow into linear fibers (bone, tendons) or they are arranged in any direction to make reticular fibers (skin). The triple strand helix and covalent cross-linking give collagen the great mechanical strength. It plays an important role in life activities such as hair growth, wound healing as well as organ supporting or body protection. Most collagen is present in the connective tissue, skin, and bones of animals
4 level Structure of protein