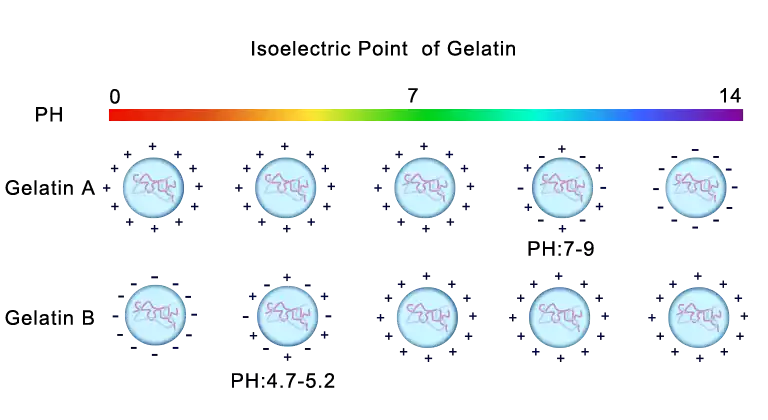
Although they are both extracted from collagen, there are some differences in their properties.
Type A gelatin is extracted from raw material in acid condition. The protonated amino groups inhibit the Maillard reaction between protein and saccharide to result in lighter color. The positively charges in molecules are from protonation, so its isoelectric point is between pH 7 and 9. The stability is good in an acidic environment, as positively charged molecules repel each other to avoid aggregation and nucleophilic attack by protons.
The molecular weight distribution is very wide: it ranges from ten kDa to several thousand kDa, and alpha chain at approximately 100 kDa is slightly abundant. The acid pretreated gelatin has a higher fat content because fat is not completely dissolved during acid pretreatment.
Type B gelatin is derived from alkali pretreated raw material. Deprotonation of amino groups and ionization of carboxyl groups give its negative charges, so the isoelectric point is between pH 4.7 and 5.2. Unlike type A gelatin, in alkaline condition, some of asparagine and glutamine will lose amino groups to become aspartic acid and glutamic acid, leading to slightly less nitrogen element than the raw material. The molecular weight distribution is relatively narrow: main component is gel strength determining alpha chains. Nonetheless, molecules over 100 KD, the primary contributors to viscosity, are more abundant than type A gelatin, so alkali-treated gelatin is usually more viscous. During alkali pretreatment, saponification almost completely removes fat. It is more stable in alkaline environment, and the reason is similar to type A gelatin.
| Gelatin Type A vs Tybe B | ||
|---|---|---|
| Type A gelatin | Type B gelatin | |
| Isoelectric point | PH 7-9 | PH 4.7-5.2 |
| Bloom value or gel strength | Similar | Similar |
| Viscosity | Low viscosity | High viscosity |
| Amino acid content | Unchanged | asparagine→aspartic acid and glutamine→glutamic acid |
| Molecular weight distribution | Uniform distribution from a dozen KDa to several thousand KDa, with more abundant 100 KDa parts | Concentrated on 100 KDa, others less |
| Color | Yellowish | More yellowish than gelatin type A |
| Impurities | More fat | Less fat |
| Stability | More stable in acid | More stable in alkali |